Table of Contents
Introduction
Insulin resistance (IR) is a growing concern, affecting millions globally and serving as a significant precursor to chronic health conditions, most notably type 2 diabetes and heart disease. Understanding the link between IR and cardiovascular health can help individuals take proactive steps to protect their heart and overall well-being.
The debate over whether insulin resistance or the lipid hypothesis better explains coronary artery disease (CAD) is an ongoing topic in medical research. Both theories have their proponents, but insulin resistance offers a compelling explanation for CAD by linking metabolic dysfunction to cardiovascular health more directly than cholesterol levels alone.
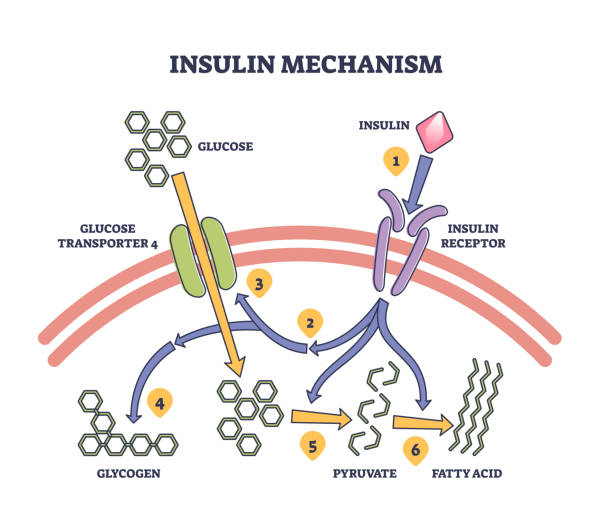
Defining and Measuring Insulin Resistance (IR)
Insulin resistance (IR) is a condition characterised by a diminished ability of the body to respond to the action of insulin, a hormone secreted by the pancreas. Insulin is crucial for regulating blood glucose levels by facilitating its uptake into cells for energy production and storage. When cells become resistant to insulin, blood glucose levels rise, prompting the pancreas to produce more insulin to maintain normal blood sugar levels. Hyperinsulinaemia often precedes the development of type 2 diabetes mellitus (T2D) and various cardiovascular complications.
Key Tissues Affected by IR
• Skeletal Muscle: The primary site for insulin-stimulated glucose uptake. In IR, muscles fail to effectively absorb glucose, contributing to elevated blood sugar levels.
• The Liver: Plays a central role in glucose production and storage. In the context of IR, the liver continues to produce glucose even when blood sugar levels are high, exacerbating hyperglycaemia.
• Adipose Tissue: Not only stores fat, but also functions as an endocrine organ. Insulin resistance in adipose tissue leads to the excess release of free fatty acids (FFAs), further impairing insulin sensitivity in other tissues.
Consequences of Insulin Resistance
• Hyperglycaemia: Elevated blood glucose levels due to reduced cellular glucose uptake.
• Hyperinsulinaemia: Persistently high insulin levels as the body attempts to counteract resistance and maintain normoglycaemia.
These conditions establish the foundation for various metabolic dysfunctions and increase the risk for cardiovascular disease (CVD).
Methods for measuring insulin resistance
Several tools and techniques have been used to assess IR.
• Fasting Insulin Test: Measures insulin levels in the blood after an overnight fast.
• HOMA-IR (Homeostatic Model Assessment of Insulin Resistance): Calculated index using fasting glucose and insulin levels. This provides an estimate of insulin sensitivity.
• Glucose Clamp Technique: The gold standard for measuring insulin sensitivity involves the controlled infusion of glucose and insulin to maintain specific blood glucose levels.
Mechanisms Linking IR to Cardiovascular Disease (CVD)
Insulin resistance contributes to cardiovascular disease through a complex network of interconnected mechanisms. Elucidating these pathways helps illustrate why individuals with IR face higher cardiovascular risks.
A. Obesity and Adipose Tissue Dysfunction
. Obesity, particularly visceral obesity (accumulation of fat around abdominal organs), is strongly associated with IR and CVD. Visceral adipose tissue (VAT) secretes bioactive molecules known as adipokines, which influence inflammation and insulin action.
. Adipokine Dysregulation: The balance of hormones such as leptin (involved in appetite regulation), resistin (pro-inflammatory), and adiponectin (improves insulin sensitivity) is disrupted. Low levels of adiponectin, and high levels of leptin and resistin, contribute to IR and inflammation.
• Lipotoxicity: Excess FFAs released from dysfunctional adipose tissue accumulates in other tissues such as the liver, muscle, and heart, promoting insulin resistance and cellular damage.
B. Inflammation and Oxidative Stress
IR is frequently accompanied by chronic low-grade inflammation that is detrimental to cardiovascular health. The immune system responds to this persistent inflammatory state, leading to the release of inflammatory cytokines such as tumour necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and C-reactive protein (CRP). These cytokines interfere with insulin signalling and promote arterial damage.
• Reactive Oxygen Species (ROS) are produced as byproducts of metabolic processes; when present in excess, ROS can lead to oxidative stress. This oxidative damage affects cells, blood vessels, and the heart, and contributes to CVD.
• Advanced glycation end products (AGEs): When sugars react with proteins or lipids, AGEs accumulate and lead to the cross-linking of collagen in blood vessels, impairing their elasticity and function.
C. Dyslipidaemia
Insulin resistance is associated with abnormal lipid metabolism, which significantly increases CVD risk.
•Elevated triglyceride level
•Low high-density lipoprotein cholesterol (HDL-C)
Insulin resistance contributes to low levels of high-density lipoprotein (HDL) cholesterol. This decrease is attributed to several factors, including the reduced activity of lipoprotein lipase, which is responsible for VLDL clearance. Additionally, the transfer of triglycerides to HDL particles via cholesteryl ester transfer protein (CETP) and subsequent breakdown by hepatic lipase leads to a reduction in plasma HDL-C and apoA-I. These processes result in the formation of small, dense HDL particles that are more rapidly cleared from circulation, further contributing to low HDL levels.
. Increased Small Dense LDL Cholesterol: The third alteration involves an increase in small, dense low-density lipoprotein (sdLDL) particles. This shift in LDL particle size is primarily driven by the increased VLDL production in the liver associated with insulin resistance. As VLDL is metabolised it contributes to the formation of LDL, particularly sdLDL. Additionally, the exchange of triglycerides for cholesteryl esters in LDL via CETP leads to triglyceride-rich LDL, which is smaller and denser. The reduced lipoprotein lipase activity further promotes the formation of sdLDL by hindering triglyceride-rich lipoprotein clearance. sdLDL particles are considered highly atherogenic due to their ability to easily penetrate the arterial wall and their susceptibility to oxidation, both of which increase cardiovascular risk.
D. Endothelial Dysfunction
The endothelium, which constitutes the thin lining of the blood vessels, plays an important role in vascular health. Insulin resistance impairs endothelial function through multiple pathways.
IR impairs the ability of blood vessels to dilate, making them more prone to damage from oxidative stress. Dysfunctional endothelium becomes more susceptible to plaque formation.
•Reduced Nitric Oxide (NO) Production: NO is essential for vessel relaxation and blood flow. Insulin resistance disrupts NO synthesis, which leads to vasoconstriction.
•Increased Vascular Permeability and Leukocyte Adhesion: This promotes inflammation and atherosclerotic plaque formation, thereby contributing to arterial stiffening.
E. Hypertension
Insulin resistance contributes to elevated blood pressure by:
•Activation of the Renin-Angiotensin-Aldosterone System (RAAS): This system regulates blood volume and pressure, but becomes hyperactive in IR, leading to vasoconstriction and fluid retention.
•Sympathetic Nervous System Overactivity: IR enhances the activity of the sympathetic nervous system, elevating the heart rate and blood pressure.
F. Myocardial Damage
The capacity of the heart to adapt to energy metabolism is disrupted by IR.
Metabolic flexibility: The heart’s ability to efficiently switch between glucose and fatty acids for energy is impaired, leading to inefficient energy production.
•Increased Substrate Availability: Elevated levels of circulating glucose and FFAs can overwhelm cells and induce cellular damage.
•Gene Expression Changes and Inflammation: Chronic IR can alter gene expression, contributing to inflammation, oxidative stress, and lipid accumulation in the cardiac muscle, potentially resulting in cardiomyopathy.
Health Conditions Associated with IR
IR is not an isolated condition but is frequently associated with other metabolic disorders.
•Type 2 Diabetes Mellitus (T2D): Prolonged IR diminishes the capacity of pancreatic beta cells to produce insulin, leading to T2D.
. Cardiovascular Disease (CVD): The multifaceted relationship between IR and heart disease manifests as an increased risk of myocardial infarction, atherosclerosis, heart failure, and stroke – increased risk due to vascular inflammation and hypertension. Chronic IR is linked to inflammation and plaque formation in blood vessels. Coronary artery disease which is accelerated by the atherogenic dyslipidemia seen in IR.
. Metabolic Syndrome (MetS): A cluster of conditions including hypertension, hyperglycaemia, excess abdominal adiposity, and abnormal cholesterol/triglyceride levels.
•Obesity: Both a contributor and consequence of IR, particularly with increased visceral adiposity.
.Metabolic Associated Fatty Liver Disease (MAFLD) and Metabolic Associated Steatohepatitis (MASH): Accumulation of hepatic lipids is associated with IR, which can progress to hepatic inflammation and damage.
•Polycystic Ovary Syndrome (PCOS) is a hormonal disorder in women linked to IR often manifesting as menstrual irregularities, infertility, and excess androgen production.
Neurological and Cognitive Disorders
. Alzheimer’s Disease – Often referred to as “type 3 diabetes” due to its strong association with IR.
. Cognitive Decline – IR may impair brain glucose metabolism, contributing to memory and learning deficits.
. Peripheral Neuropathy – Common in individuals with IR, even without overt diabetes.
Inflammatory and Immune Conditions
. Chronic Inflammation – IR drives systemic inflammation through dysregulated cytokine release.
. Autoimmune Diseases – IR has been linked to an increased risk of conditions like rheumatoid arthritis.
. Psoriasis – Strongly associated with metabolic syndrome and IR.
Reproductive and Hormonal Disorders
. Gestational Diabetes – IR worsens during pregnancy, increasing risks to mother and baby.
. Erectile Dysfunction – A result of endothelial dysfunction and vascular issues related to IR.
Renal and Hepatic Disorders
. Chronic Kidney Disease (CKD) – IR contributes to glomerular dysfunction and kidney damage.
. Hyperuricemia and Gout – IR reduces renal clearance of uric acid.
. Cancer – Increased risk of certain cancers (e.g., breast, colorectal, pancreatic) due to hyperinsulinemia.
. Sarcopenia – Reduced muscle mass and strength may co-occur with IR in ageing populations.
Management Strategies for IR and CVD Risk Reduction
Effective management of IR requires a multifaceted approach involving lifestyle modifications, dietary interventions, and pharmacological therapies.
A. Non-Pharmacological Interventions
Dietary Modifications
•Low glycaemic index (GI) and low-carbohydrate diets: Diets that emphasise foods with a low GI contribute to the maintenance of stable blood glucose levels and reduce insulin spikes.
•High-fibre diet: promotes satiety, decelerates glucose absorption, and supports gut health.
•Balanced Macronutrients: Ensuring an appropriate balance of proteins, carbohydrates, and fats contributes to the maintenance of metabolic health.
•Plant-Based Proteins and Whole Grains: Promote insulin sensitivity and mitigate inflammation.
•Mediterranean Diet: Abundant in fruits, vegetables, whole grains, lean protein, and healthy fats, such as olive oil. This dietary pattern enhances insulin sensitivity and reduces the risk of CVD.
. Low-carb high-fat diets have also shown to improve IR. Populations with minimal insulin resistance (e.g., traditional hunter-gatherers) tend to have low rates of CAD despite dietary cholesterol or saturated fat intake.
•Calorie restriction and IF: Techniques such as early time-restricted feeding (TRF) and nutritional ketosis can promote metabolic flexibility, enhance insulin sensitivity, and facilitate weight management.
Regular Exercise
•Aerobic Exercise: Engaging in activities such as walking, running, and cycling for a minimum of 150 minutes of moderate-intensity or 75 minutes of vigorous exercise per week enhances glucose uptake by muscle cells.
•Resistance Training: Strength training augments muscle mass, which contributes to improved glucose utilisation and insulin sensitivity.
Other Lifestyle Factors
.Weight Management: Reducing excess body weight, particularly visceral fat, is crucial for reversing IR.
•Stress Management: Elevated stress levels can lead to increased cortisol levels, which exacerbate insulin resistance. Practices such as mindfulness, yoga, and deep-breathing exercises can facilitate stress management.
Adequate Sleep: Poor sleep quality and duration can impair insulin sensitivity. Ensuring 7-9 hours of quality sleep per night is essential for metabolic health.

B. Pharmacological Interventions
When lifestyle modifications alone are insufficient, pharmacological interventions can play a significant role in managing insulin resistance.
- Metformin: Often the first-line treatment for T2D, metformin inhibits hepatic glucose production and enhances peripheral glucose uptake. It contributes to improved insulin sensitivity and reduced blood glucose levels.
- Thiazolidinediones (TZDs): Drugs such as pioglitazone activate peroxisome proliferator-activated receptor-gamma (PPAR-γ), which enhances insulin sensitivity by augmenting glucose uptake in muscle and adipose tissues. However, they may have adverse effects such as weight gain, fluid retention, and heart failure risk.
- GLP-1 receptor agonists: Liraglutide and semaglutide mimic glucagon-like peptide-1, a hormone that enhances insulin secretion, suppresses glucagon, and improves insulin sensitivity. They also promote weight loss, which further aids in reducing IR and CVD risk.
- SGLT2 Inhibitors: These drugs, such as empagliflozin and dapagliflozin, block sodium-glucose cotransporter 2 in the kidneys, leading to increased glucose excretion in the urine. They contribute to lowering blood sugar and body weight and have demonstrated protective effects on the heart and kidneys.
Note: While these medications assist in managing blood glucose levels and improving insulin sensitivity, they do not directly treat IR itself. Lifestyle modifications remain a cornerstone of IR management.
Emerging Research Areas
Research on IR and its association with cardiovascular health continues to expand, elucidating new insights and potential therapeutic targets.
Elucidating the complex pathways that lead to IR is essential for the development of novel treatments. Current research focuses on the roles of specific enzymes, signalling molecules, and genetic factors that contribute to this condition.
Traditional measurements such as fasting glucose and HOMA-IR may not capture early changes in insulin sensitivity. Researchers are investigating new biomarkers, such as adiponectin, leptin-to-adiponectin ratio, and inflammatory cytokines, which could facilitate earlier and more accurate detection of IR.
IR in Children
With increasing rates of childhood obesity, IR in the paediatric population has become a growing concern. Research is investigating the long-term effects of early onset IR on metabolic and cardiovascular health in adulthood, and how interventions in childhood can mitigate these risks.
Role of Branched-Chain Amino Acids (BCAAs)
Elevated levels of BCAAs (leucine, isoleucine, and valine) have been associated with IR. Current studies are examining how BCAAs contribute to metabolic disturbances and whether modifying their intake can improve insulin sensitivity.
Mitochondrial Dysfunction, miRNAs, and autophagy
The mitochondria play a pivotal role in energy metabolism. Dysfunctional mitochondria can lead to inefficient energy production and increased oxidative stress, exacerbating IR. Additionally, microRNAs (miRNAs), which are small RNA molecules that regulate gene expression, are being studied for their potential to influence insulin sensitivity. Autophagy, the cellular cleaning process of the body, may also play a protective role in maintaining insulin sensitivity and is a subject of ongoing research.
Myokines, Hepatokines, and Adipocytokines
Proteins and hormones released by the muscle (myokines), liver (hepatokines), and adipose tissue (adipocytokines) are gaining attention as potential regulators of insulin sensitivity and metabolic health. Examples include irisin, fetuin-A, and fibroblast growth factor 21 (FGF21).
New Pharmacological Agents
Researchers are currently investigating next-generation drugs that target IR pathways more effectively. Agents that can modulate AMP-activated protein kinase (AMPK) activity, sirtuins, and inflammation pathways hold promise for the improved management of IR.

Conclusion
IR is a complex and multifaceted condition that significantly contributes to the risk of cardiovascular diseases. It involves intricate interactions among metabolic, inflammatory, and hormonal pathways, ultimately leading to systemic effects that adversely affect cardiovascular health. Addressing IR requires a comprehensive approach, primarily focusing on lifestyle modifications such as a balanced diet, regular exercise, weight management, stress reduction, and adequate sleep. Pharmacological options can complement these changes when necessary but are most effective as part of an overall treatment strategy.
Emerging research continues to enhance our understanding of IR revealing new mechanisms, potential biomarkers, and novel treatment options. This ongoing exploration is essential to develop more effective interventions and improve health outcomes. By maintaining a proactive approach towards managing IR, individuals can significantly lower their risk of developing cardiovascular diseases and enhance their overall quality of life.
This article is not intended to replace professional medical advice. If you have specific health concerns or conditions, consult with a healthcare professional for personalised guidance.
Disclaimer: The information provided in this article is for educational purposes only and should not be considered as medical advice. Always consult with a healthcare professional before making any changes to your diet or lifestyle.



